Objectives
Human embryonic stem cells (hESCs) serve as an attractive cell source for generating functional blood cells and hold great potential for cell-based therapy in future clinical applications. However, the underlying molecular mechanisms that regulate hESC differentiation into hematopoietic cells remain elusive. Therefore, understanding hESC differentiation is essential for the acquisition of sufficient and functional cells in vitro for successful clinical translation.
Materials and Methods
ELTD1 was identified as a potential mediator of hESC hematopoietic differentiation by genome-wide gene profiling. ELTD1-deleted human embryonic stem cell (hESC) lines were constructed by the iCRISPR/Cas9 technology and were used to undergo hematopoietic differentiation. Flow cytometry, quantitative RT-PCR, immunofluorescence, and RNA sequencing were used for phenotype and molecular mechanism assessment.
Results
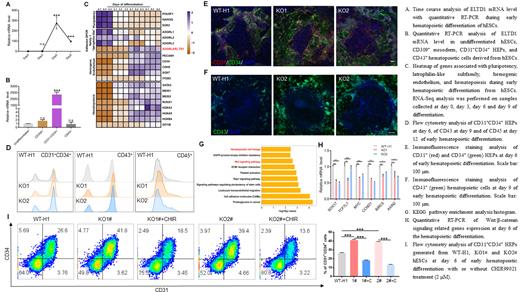
Real-time PCR analysis and bioinformatic studies demonstrated that the dynamic expression of ELTD1 parallels the hemogenic endothelial progenitors-related genes expression and subsequently decreased as they differentiate into hematopoietic cells ( Figure A, C), leading us to speculate that it might play a role in hESC early hematopoietic differentiation. To further confirm these results, stage-specific cell populations at different time points were sorted to document the cell-type specificity of ELTD1. As shown in Figure B, ELTD1 was exclusively expressed in CD31 +CD34 + HEP populations. Loss-of-function studies revealed that ELTD1 deletion promoting the induction of CD31 +CD34 + HEPs at day 6, thereby promoting endothelial-to-hematopoietic transition to generate more CD43 +/CD45 + hematopoietic cells, and the results were confirmed by flow cytometry and immunofluorescence analyses ( Figure D, E and F). To dissect the molecular mechanism by which ELTD1 regulates hESC hematopoietic differentiation, the differentiated cells at day 6 were used to perform the RNA-Seq. KEGG analysis showed that hematopoietic cell lineage-related genes and Wnt signaling pathway were prominently enriched in ELTD1-deleted cells compared to WT-H1, consistent with the enhancement of HEPs and hematopoietic cells generation caused by ELTD1 deletion ( Figure G). Moreover, decreased expression of Wnt target genes: SOX17, TCF7L1, MYC, CCND1, BIRC5, and AXIN2 was further validated by quantitative RT-PCR analysis ( Figure H). To determine whether the downregulation of Wnt/β-catenin signaling is required for ELTD1 deletion-mediated enhancement of early hematopoietic differentiation from hESCs, we treated ELTD1-KO1# and ELTD1-KO2# hESCs with the Wnt agonist CHIR99021. As expected, treatment with CHIR99021 inhibited the generation of CD31 +CD34 + HEPs compared to ELTD1 deleted alone at day 6, which was validated by flow cytometry ( Figure I).
Conclusions
We identified ELTD1 as a novel regulator of hematopoietic differentiation, which may act via Wnt/β-catenin signaling, and provided new insights into understanding the mechanism of human hematopoiesis.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal